Impactful therapeutic outcomes
Functional Outcomes of PoNS Therapy®
Gait deficit results from impairment of neural mechanisms that deliver the signal to, and through, the spinal cord. Physical therapy is known to provide therapeutic benefits to MS patients with gait impairment. However, physical therapy alone may not be enough to provide a long-term functional improvement.
As observed in PoNS® clinical trials, the potential neuromodulation effect of physical therapy, observed in the sham group and showing increased activity in the bilateral premotor cortex areas from baseline, may be responsible for a short-term clinically meaningful improvement in gait. However, since physical therapy alone is not likely to induce neuroplastic effects, this may contribute to a decline of function over the treatment period.
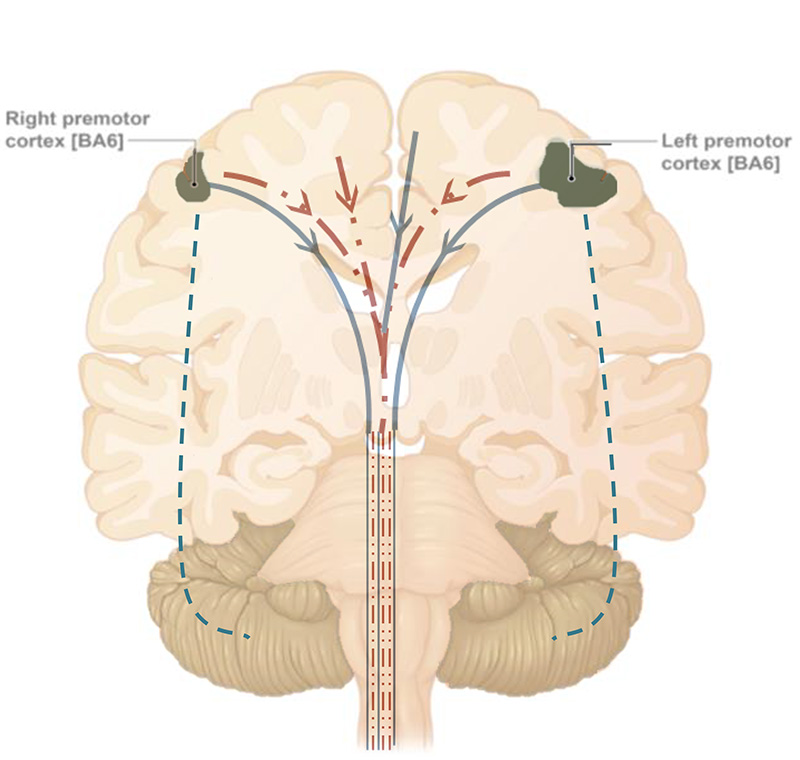
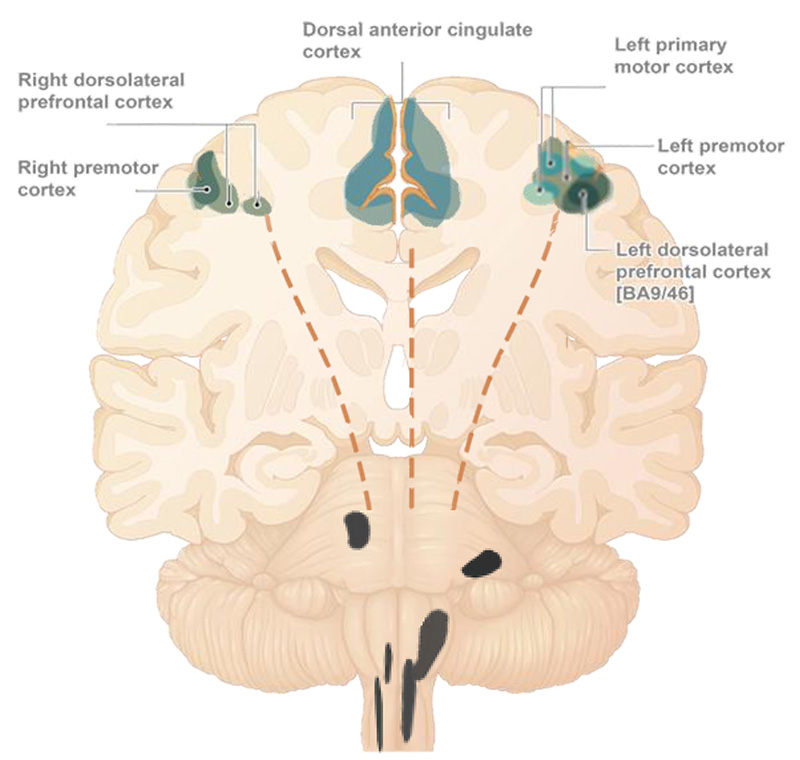
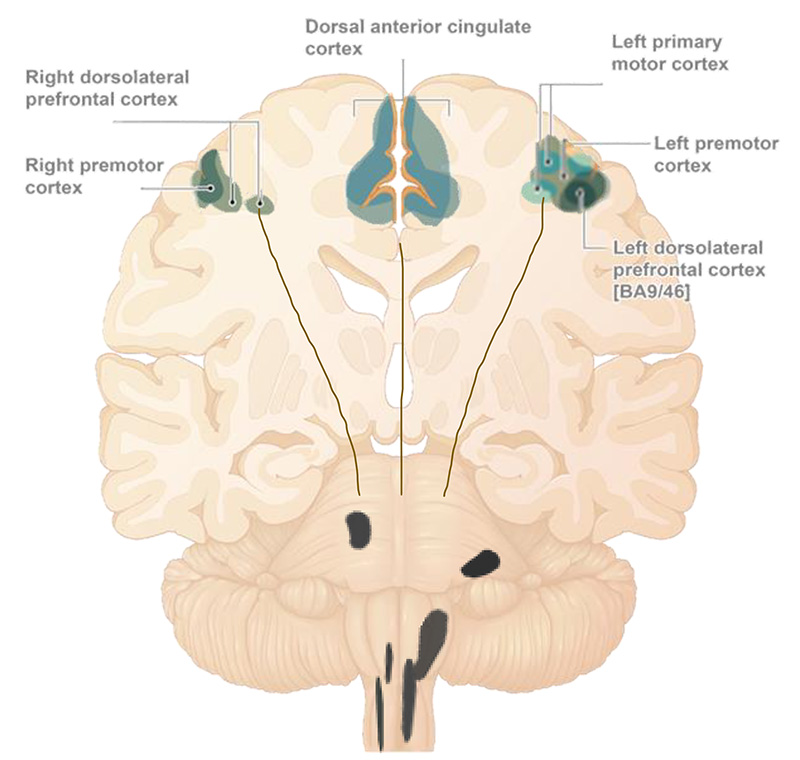
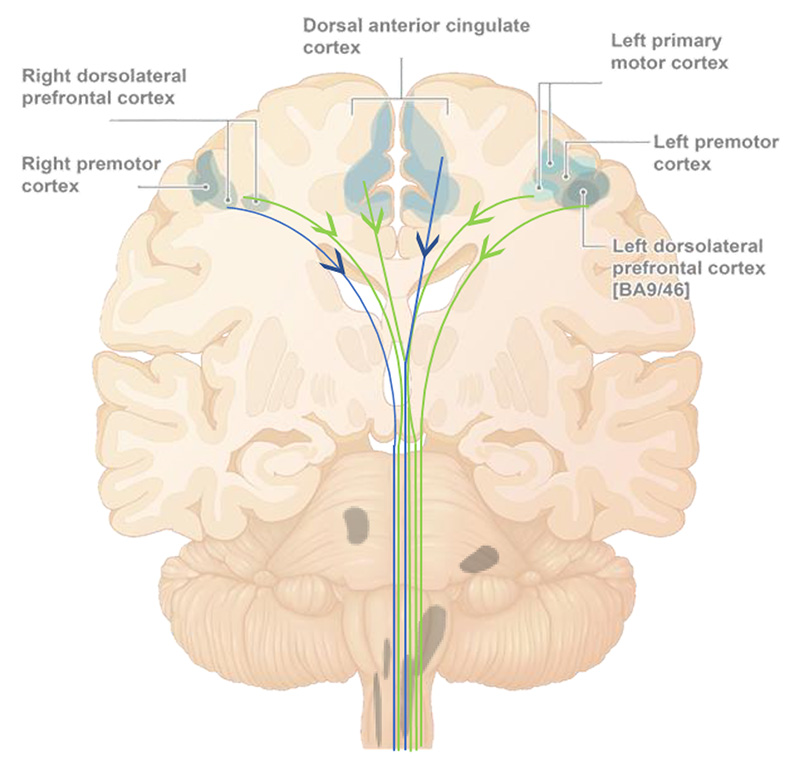
Consolidation of compensatory processes resulting from engaging neuroplasticity mechanisms by daily treatment, may be responsible for promoting functional improvement (peaking at week 10) and maintaining it through the end of the treatment period (14 weeks).
Clinical Benefits and Real-World Evidence
Real world data consolidate the evidence on clinical benefits and therapeutic outcomes with 14 weeks of PoNS Therapy in individuals with gait and balance deficit. A retrospective analysis of RWE effectiveness data demonstrated clinically and statistically significant improvements in gait for individuals with MS using PoNS as reflected by improved FGA total scores. Some individuals have reported headaches, fatigue, and excess salivation. Excess salivation during training sessions often occurs but generally improves as patients get used to wearing the mouthpiece.
Click here to view the RWE Poster Presentation.
Healthcare providers interested in additional medical/scientific information about PoNS® can submit a Medical Information Request Form. A Medical Affairs representative will respond within 24 hours to provide the requested information.
REFERENCES:
Tyler ME, Kaczmarek KA, Rust KL, Subbotin AM, Skinner KL, Danilov YP. Non-invasive neuromodulation to improve gait in chronic multiple sclerosis: a randomized double blind controlled pilot trial. J Neuroeng Rehabil. 2014;11:79.
Danilov Y, Skinner K, Tyler M (2015). Brain Neurotrauma: Molecular, Neuropsychological, and Rehabilitation Aspects. CRC Press/Taylor & Francis.
